Overview - Parasitism and Natural Enemies
Bumblebees (Bombus spp.) host a wide variety of parasites, all of which impose varying fitness costs on individuals and colonies. Nonetheless, little is currently understood about the importance of parasites in the population dynamics of bumblebees.
Among the better-studied parasites are the microsporidian Nosema bombi (Kingdom: Fungi) and the Trypanosome Crithidia spp. (Kingdom: Protista). Nosema is an obligate intracellular parasite that causes systemic infections in bumble bees (i.e. it can invade all tissues), and Crithidia is an intestinal parasite. Both Nosema and Crithidia are transmitted by an oral-fecal route, and both negatively impact bumblebee fitness. Once thought to be benign, Crithidia has since been shown to increase worker mortality, inhibit colony founding and growth, and impair learning in individual foragers. The effects of Nosema have been harder to establish, but inoculation experiments have definitively demonstrated that Nosema infections can reduce worker survival and inhibit colony growth. Shared use of flowers can lead to horizontal transmission of parasites between worker bees; thus host-sharing is a likely mechanism of both inter-colony and inter-species disease transfer. Learn more about these parasites!
A variety of parasitoids [what is a parasitoid?] also use bumblebees as hosts, including a solitary endoparasitoid that has been the subject of ongoing study in my own laboratory - the conopid fly (Diptera, Conopidae). Several species of conopid flies attack adult bumblebees and implant individual eggs into the abdominal cavity of bumblebee hosts. Typically, a single endoparasitic larva grows within the bee over the course of 10 to 12 days, feeding on gut tissue and eventually killing the bee. More information about the conopid fly life cycle can be found here. Conopid larvae change the foraging behavior of their hosts: infected individuals have an impaired ability to handle complex flowers and are less likely to collect pollen, which may have negative impacts on colony growth. By shortening the lifespan of bumblebee hosts conopids may also have an impact on the health of a colony, as overall colony size is important for resource return and thus reproduction.
My research has focused on detecting parasitism prevalence and infection intensity among bumblebee host species and has more deeply investigated the interaction between conopid flies and their bumblebee hosts. The overarching goal of these endeavors is to reveal possible differences in susceptibility and tolerance (i.e., vulnerability) to parasitic infections, which may have implications for population dynamics and community-level species composition in the long-term.
Among the better-studied parasites are the microsporidian Nosema bombi (Kingdom: Fungi) and the Trypanosome Crithidia spp. (Kingdom: Protista). Nosema is an obligate intracellular parasite that causes systemic infections in bumble bees (i.e. it can invade all tissues), and Crithidia is an intestinal parasite. Both Nosema and Crithidia are transmitted by an oral-fecal route, and both negatively impact bumblebee fitness. Once thought to be benign, Crithidia has since been shown to increase worker mortality, inhibit colony founding and growth, and impair learning in individual foragers. The effects of Nosema have been harder to establish, but inoculation experiments have definitively demonstrated that Nosema infections can reduce worker survival and inhibit colony growth. Shared use of flowers can lead to horizontal transmission of parasites between worker bees; thus host-sharing is a likely mechanism of both inter-colony and inter-species disease transfer. Learn more about these parasites!
A variety of parasitoids [what is a parasitoid?] also use bumblebees as hosts, including a solitary endoparasitoid that has been the subject of ongoing study in my own laboratory - the conopid fly (Diptera, Conopidae). Several species of conopid flies attack adult bumblebees and implant individual eggs into the abdominal cavity of bumblebee hosts. Typically, a single endoparasitic larva grows within the bee over the course of 10 to 12 days, feeding on gut tissue and eventually killing the bee. More information about the conopid fly life cycle can be found here. Conopid larvae change the foraging behavior of their hosts: infected individuals have an impaired ability to handle complex flowers and are less likely to collect pollen, which may have negative impacts on colony growth. By shortening the lifespan of bumblebee hosts conopids may also have an impact on the health of a colony, as overall colony size is important for resource return and thus reproduction.
My research has focused on detecting parasitism prevalence and infection intensity among bumblebee host species and has more deeply investigated the interaction between conopid flies and their bumblebee hosts. The overarching goal of these endeavors is to reveal possible differences in susceptibility and tolerance (i.e., vulnerability) to parasitic infections, which may have implications for population dynamics and community-level species composition in the long-term.
Patterns of parasitism in wild bumblebee populations in northern VA
Malfi, R.L. & Roulston, T.H. (2014)
Malfi, R.L. & Roulston, T.H. (2014)
 Bombus fervidus on bleeding hearts.
Bombus fervidus on bleeding hearts.
Study Objective and Methods: In 2011, I examined the prevalence of three parasitic organisms in wild populations of bumblebees collected from meadows (including the Native Plant Meadow at Blandy Experimental Farm) throughout the northern Shenandoah Valley and Piedmont regions of Virginia. I examined over 800 bees, collected between June 1 and July 31, for infections of Nosema and Crithidia, as well as the presence of parasitoid conopid fly larvae. In addition to assessing the prevlance of these parasites in bumblebee hosts, I also wanted to better understand factors that may influence patterns of both infection prevalence (% individuals infected) and intensity (# parasites per individual host), including (1) bee species identity, (2) bee body size, and (3) bee phenology (i.e., timing of seasonal activity).
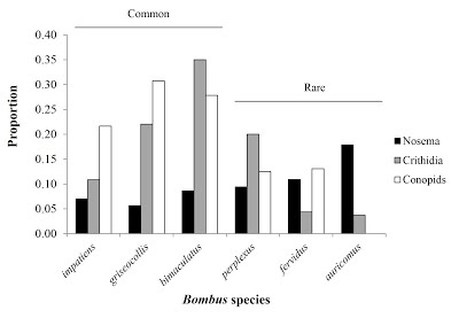
Results and Importance: This research provides important baseline information about the relative abundance of both bumble bee species and their parasites in the geographical area of study, and illuminates differences in parasitic infections among species that warrant additional investigation. Specifically, we found strong evidence that parasitic infections vary among host bumblebee species according to their relative rarity (Figure 1). This work is one of few studies to document the prevalence and patterns of parasitism in North American bumblebees, and it is one of even fewer to examine host-parasite relationships in bumblebee populations of the eastern U.S. This study found that locally rare bumblebee species were more likely to be infected by Nosema, a parasitic fungal pathogen that has been implicated in the decline of several North American bumblebees in recent decades. Nosema infections also tended to be more intense in rare species than in their abundant congeners. The two rarest species in this study (B. fervidus and B. auricomus) have recently been identified as species that are in decline (Colla et al. 2012), and we found Nosema to be most prevalent in these bees relative to others in the local community. In comparison to other North American surveys of bumblebees, we document some of the highest overall rates of Nosema and Crithidia infections yet recorded for the continent. For a full account of the study and my results, see my recent publication in Ecological Entomology or email me a request for a reprint.
Results and Importance: This research provides important baseline information about the relative abundance of both bumble bee species and their parasites in the geographical area of study, and illuminates differences in parasitic infections among species that warrant additional investigation. Specifically, we found strong evidence that parasitic infections vary among host bumblebee species according to their relative rarity (Figure 1). This work is one of few studies to document the prevalence and patterns of parasitism in North American bumblebees, and it is one of even fewer to examine host-parasite relationships in bumblebee populations of the eastern U.S. This study found that locally rare bumblebee species were more likely to be infected by Nosema, a parasitic fungal pathogen that has been implicated in the decline of several North American bumblebees in recent decades. Nosema infections also tended to be more intense in rare species than in their abundant congeners. The two rarest species in this study (B. fervidus and B. auricomus) have recently been identified as species that are in decline (Colla et al. 2012), and we found Nosema to be most prevalent in these bees relative to others in the local community. In comparison to other North American surveys of bumblebees, we document some of the highest overall rates of Nosema and Crithidia infections yet recorded for the continent. For a full account of the study and my results, see my recent publication in Ecological Entomology or email me a request for a reprint.
Conopid flies cause "grave-digging" behavior in bumblebee hosts... do some host species make better victims?
Malfi, R.L., Davis, S.E., & Roulston, T.H. (2015)
Malfi, R.L., Davis, S.E., & Roulston, T.H. (2015)
Study Objective and Methods: A study conducted in 1994 by Dr. Christine Muller describes a fascinating (or horrifying, depending on your perspective) phenomenon in which bumblebees harboring the endoparasitic larvae of conopid flies buried themselves in soil just prior to their own death. Through this study, which featured one European bumblebee host species (Bombus terrestris), Muller experimentally concluded that this behavior, of no consequence to the dying host, was beneficial to the developing parasitoid. A conopid fly larva develops within its host over the course of 10-12 days, gradually consuming the host's innards and eventually killing the host bee at the end of that time period. The larva then pupates and overwinters within the exoskeleton (or "mummy") of its host, emerging as an adult fly the following spring. Muller found that buried pupae were less likely to experience predation and that pupae that overwintered in the soil produced larger adult flies with fewer wing deformities than those that overwintered at the ground surface. Collectively, evidence from her study indicates that flies induce self-burial (or "grave-digging") behavior in their host bumblebees to improve their own survival and adult fitness. Such an interaction is called an adaptive manipulation because the parasite benefits from manipulating its host's behavior.
Muller's study was the only published account of this adaptive manipulation, and led us to ask the following questions. Does this manipulation of host behavior exist across multiple bumblebee host species? Do we see this behavior in our local, North American bumblebees? And most importantly - are conopids capable of manipulating all of their utilized host species equally? My REU student, Staige E. Davis (University of Virginia), and I investigated these questions in the summer of 2012.
For six weeks, starting on June 11, we collected worker bees (non-reproductive females) of the three most locally common bumblebee species (B. bimaculatus,B. griseocollis, and B. impatiens) weekly from naturally flowering areas at Blandy Experimental Farm. Bees were then placed into clear plastic tanks with 15 cm of loose topsoil covered in a thin layer of leaves, sticks, and rocks following the design that Muller (1994) used. Each tank held up to 20 bees of mixed species. Bees were fed artificial nectar ad libidum. When all the bees in a tank had naturally expired, we excavated the tank and categorized the death location of each bee as being (1) on the surface or (2) buried in the soil. Once bees had been pulled out of tanks, we inspected them for conopid larvae/pupae. In the third and final instar of conopid development, the larva grows to such a size that it fills the abdominal cavity of its host bee, making it readily apparent whether the bee is parastized.
For six weeks, starting on June 11, we collected worker bees (non-reproductive females) of the three most locally common bumblebee species (B. bimaculatus,B. griseocollis, and B. impatiens) weekly from naturally flowering areas at Blandy Experimental Farm. Bees were then placed into clear plastic tanks with 15 cm of loose topsoil covered in a thin layer of leaves, sticks, and rocks following the design that Muller (1994) used. Each tank held up to 20 bees of mixed species. Bees were fed artificial nectar ad libidum. When all the bees in a tank had naturally expired, we excavated the tank and categorized the death location of each bee as being (1) on the surface or (2) buried in the soil. Once bees had been pulled out of tanks, we inspected them for conopid larvae/pupae. In the third and final instar of conopid development, the larva grows to such a size that it fills the abdominal cavity of its host bee, making it readily apparent whether the bee is parastized.
Results and Importance: We found that grave-digging behavior was indeed present in the bumblebee host species examined, providing the first account of this manipulative behavior in North America. All pupae were overwintered artificially; all adult flies that emerged were identified as the species Physocephala tibialis by Dr. Joel Gibson at the University of Guelph. Our primary finding is that P. tibialis is differentially capable of inducing this behavior depending on the identity of its host. In other words, not all hosts may be equally suitable for conopid fly development. This information is valuable not only for furthering our understanding of conopid fly-bumblebee interactions, but for understanding the selective pressures that govern the evolution of host-parasitoid relationships more generally. You can read more about our findings in our recent publication in Animal Behaviour which is now available online through ScienceDirect. This work was also described in a National Geographic blog post by Ed Yong.
The enemy within - do bumblebee species exhibit differential physiological resistance to conopid flies?
Davis, S.E., Malfi, R.L., & Roulston, T.H. (2015)
Davis, S.E., Malfi, R.L., & Roulston, T.H. (2015)

Study Objective and Methods: In 2011, I surveyed free-flying bumblebees from several sites across northern VA for the presence of parasites, including parasitoid conopid fly larvae. In that year, collected individuals were placed into a deep freezer upon collection from the field. I later dissected these specimens that were "frozen in time." In this year, we found that infection rates differed among species, with more common species being infected by conopids more frequently. The three most abundant hosts species (B. impatiens, B. griseocollis, andB. bimaculatus), were not significantly different in the rate at which they were parasitzed by conopids.
The following year, my student, Staige Davis, and I once again surveyed bumblebees for conopids. Only this time, we collected bees from the field and kept them alive in the laboratory until they naturally expired. This allowed conopid larvae the opportunity to complete their development within infected hosts. Using this protocol, we were rather surprised to find that the rate of conopid parasitism was unexpectedly low (13%) in the Brown-Belted Bumblebee (B. griseocollis), the species that had highest documented rate of infection (33%) in the previous year! What did this mean? We hypothesized that the apparent drop in conopid parasitism seen in this host species between two consecutive years (2011-2012) of study indicated that all three bumblebee species are initially parasitized at roughly the same rate, but that the Brown-Belted Bumblebee might be a resistant host, capable of impeding the development of conopid fly larvae.
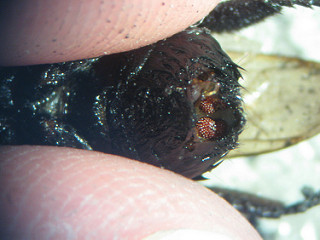
Staige returned to Blandy the following summer, and we tested this hypothesis together. We conducted two experiments. In the first experiment, we tested whether the strength of a generalized immune response to foreign entities, called the encapsulation response, varied in intensity across three bumblebee hosts (including the Brown Belted Bumblebee). In insects, host cells that recognize foreign entities (e.g. parasitoid eggs or larvae) will deposit melanin on the invader and simultanously release cytotoxic chemicals; if successful, this response kills the invader through a combination of asphyxiation and cell death. We tested the intensity of this response by placing 1-mm nylon implants in bumblebees for 2 hours, freezing these bees, dissecting out the implants, and quantifying the degree of melanization (darkened surface area) on the implants. In the second experiment, we captured bees of our target host species during the peak weeks of conopid infection and housed these individuals in the laboratory until their natural expiration. Immediately upon death, we dissected bees to determine if they harbored a conopid. This second experiment was intended to reveal potential differences in the rate of conopids successfully pupating in our target bumblebee host species.
The following year, my student, Staige Davis, and I once again surveyed bumblebees for conopids. Only this time, we collected bees from the field and kept them alive in the laboratory until they naturally expired. This allowed conopid larvae the opportunity to complete their development within infected hosts. Using this protocol, we were rather surprised to find that the rate of conopid parasitism was unexpectedly low (13%) in the Brown-Belted Bumblebee (B. griseocollis), the species that had highest documented rate of infection (33%) in the previous year! What did this mean? We hypothesized that the apparent drop in conopid parasitism seen in this host species between two consecutive years (2011-2012) of study indicated that all three bumblebee species are initially parasitized at roughly the same rate, but that the Brown-Belted Bumblebee might be a resistant host, capable of impeding the development of conopid fly larvae.
Staige returned to Blandy the following summer, and we tested this hypothesis together. We conducted two experiments. In the first experiment, we tested whether the strength of a generalized immune response to foreign entities, called the encapsulation response, varied in intensity across three bumblebee hosts (including the Brown Belted Bumblebee). In insects, host cells that recognize foreign entities (e.g. parasitoid eggs or larvae) will deposit melanin on the invader and simultanously release cytotoxic chemicals; if successful, this response kills the invader through a combination of asphyxiation and cell death. We tested the intensity of this response by placing 1-mm nylon implants in bumblebees for 2 hours, freezing these bees, dissecting out the implants, and quantifying the degree of melanization (darkened surface area) on the implants. In the second experiment, we captured bees of our target host species during the peak weeks of conopid infection and housed these individuals in the laboratory until their natural expiration. Immediately upon death, we dissected bees to determine if they harbored a conopid. This second experiment was intended to reveal potential differences in the rate of conopids successfully pupating in our target bumblebee host species.
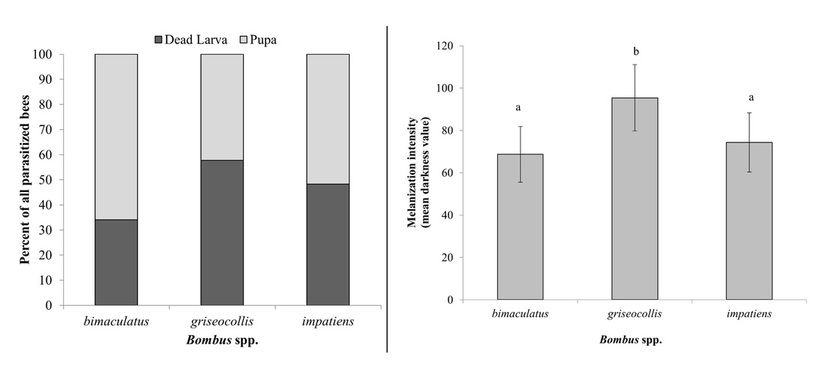
Results and Importance: Our study generated strong evidence that the Brown-Belted Bumblebee (B. griseocollis), in comparison to two other host species, is a more resistant host owing to its ability to physiologically resist conopid fly "infections". The Brown-Belted Bumblebee had a stronger encapsulation response, more frequently melanized larvae, and was more likely to kill a conopid larva prior to its own death in the lab. We also found that the correspondence between melanization/encapsulation response intensity and conopid failure were not perfectly matched across host species. Conopid larvae were just as likely to perish in the Common Eastern Bumblebee (B. impatiens) as they were in the Brown-Belted Bumblebee, even though the latter was shown to have a more intense immune response. In the case of B. impatiens, larvae were generally alive at the time of host death, indicating that premature host death was the primary cause of conopid failure (vs. failure resulting from host immune defenses). Given that larvae were frequently found dead in B. griseocollis without evidence of melanization, it is possible that some other aspect of immune function correlated with encapsulation response intensity is responsible for impeding larval development. It is also possible that some combination of physiological immune defense and behavioral defense (e.g. self-medication via dietary choices).
The question remains: why is the Brown-Belted Bumblebee a strong encapsulator? Having a strong standing immune response is energetically costly; presumably there is selective pressure acting on this host to maintain this defense. We hypothesize that close phenological overlap between the Brown-Belted Bumblebee and peak conopid activity, at least in this system, could be one driver. Additionally, this bee tends to have fewer, longer-lived workers (vs. more, shorter-lived workers), meaning that the value of each individual to the success of the colony might be higher. Our own study showed that the Brown-Belted Bumblebee was the only one of the three host species to have its lifespan in the laboratory cut short by parasitism, providing support for this notion. For more details about this study, please see our publication in Oecologia!
Assessing risk of parasitism by conopids using radio frequency technology
Malfi, R.L., Roulston, T.H., Stuligross, C., McIntosh, S., & Bauer, L.
Malfi, R.L., Roulston, T.H., Stuligross, C., McIntosh, S., & Bauer, L.

Coming soon!
For a taste of what's in store, check out a "Roadside Science" blog post on our research from science writer Carolyn Beans.
Coming soon!
For a taste of what's in store, check out a "Roadside Science" blog post on our research from science writer Carolyn Beans.